Psoriasis Study – Injections for Patients 18 Years Old and Older
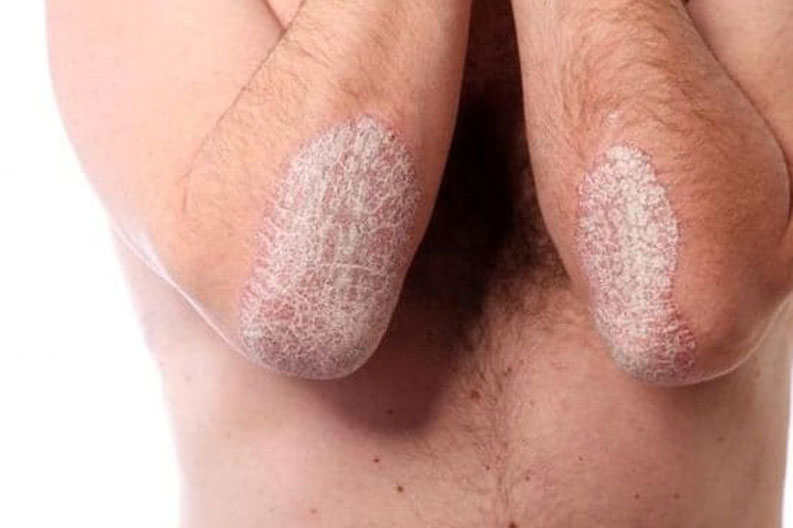
NEW study for adults 18 years and older with a diagnosis of psoriasis. Must have history of at least 6 months on Consyntex or Talz. No prior use of Skyrizi. No investigational drug use within 30 days. No major surgeries within 12 weeks, or major planned surgeries. You are being asked whether you would like to voluntarily participate in a research study of a drug called risankizumab (SkyriziTM).
This research study involves the use of an approved drug called risankizumab (SkyriziTM) to treat moderate to severe psoriasis. This study is being run in approximately 50 research centers in 9 countries and is expected to enroll approximately 250 subjects.
If you decide to participate, you will be in this study for up to 64 weeks. The study comprises 3 periods: There will be a 30-day Screening Period, a 52-week open-label study period and a 20-week follow-up period following the last dose of study drug scheduled at Week 40 visit (weeks 40-52 of the open-label period will also be weeks 1-12 of the follow-up period). The open-label period means that you, your study doctor and AbbVie know that what study treatment you are receiving.
You may need to come in for additional (unscheduled) visits if necessary, as determined by your study doctor. If you are eligible to receive study treatment, you will receive 2 injections of active risankizumab 75 mg (150 mg total dosage) subcutaneously (SC) at Weeks 0 and 4 visits, and then every 12 weeks (q12w) until the last dose at Week 40 visit. At any time, you may choose to discontinue from the study and end study participation early, or your study doctor may discontinue your participation for any reason at any time.
AbbVie is sponsoring this study. Being in this study does not replace your regular medical care. AbbVie may stop this study prematurely, either in its entirety or at any study site, for reasonable cause, provided that written notice is submitted in advance of the intended study stop.







